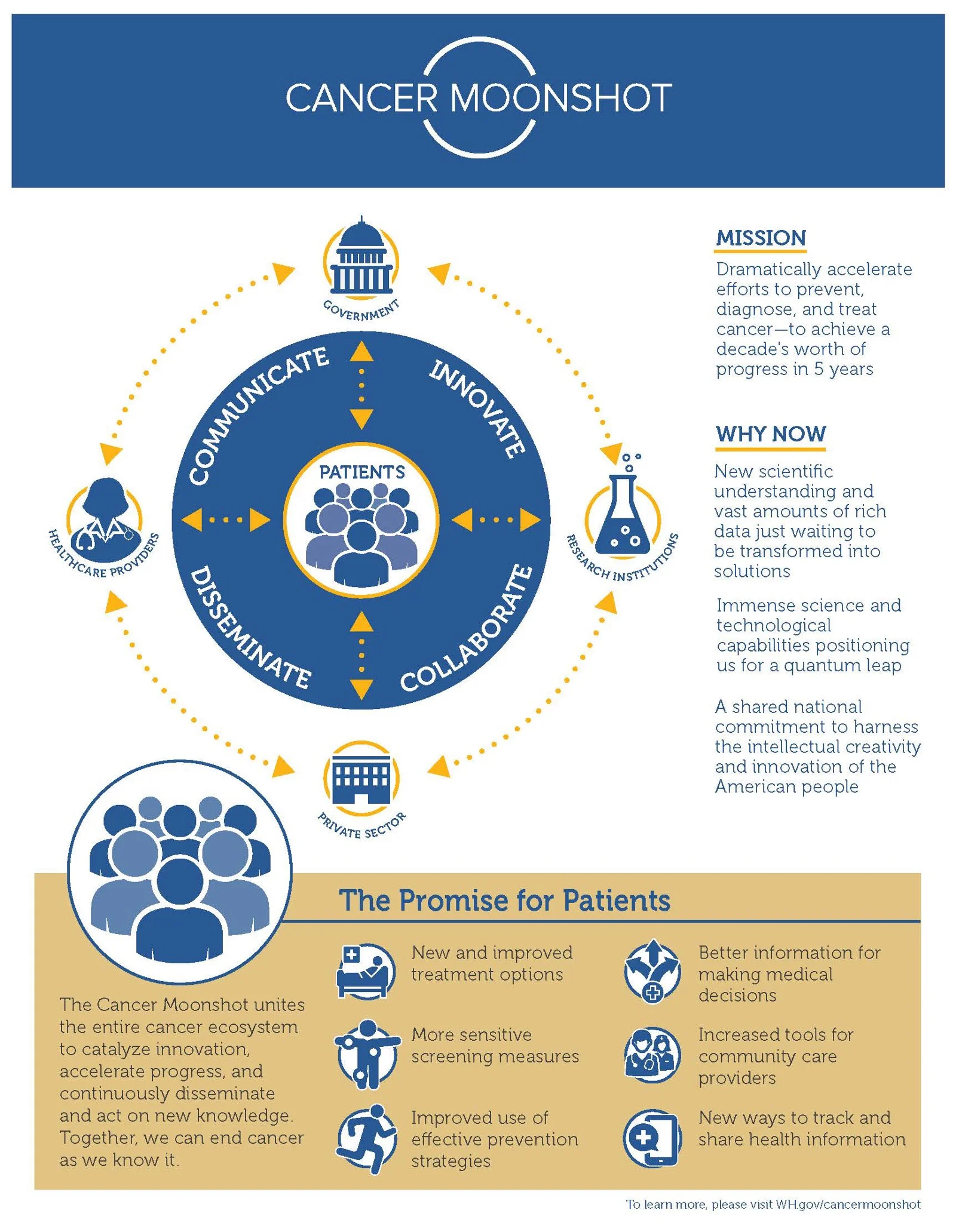
By Jenny Carlson
Definition of ‘‘Waters of the United States’’—Recodification of Pre-Existing Rules
AGENCY: Department of the Army, Corps of Engineers, Department of Defense; and Environmental Protection Agency(EPA).
ACTION: Proposed rule.
I. What is the overarching issue?
The 1972 Clean Water Act gave the federal government authority to limit pollution to both major bodies of water (i.e. Chesapeake Bay) and to streams and wetlands that drain into those bodies of water. However, in 2001 and 2006, two Supreme Court decisions resulted in legal confusion on whether or not the federal government has the authority to regulate smaller streams, headwaters, and wetlands.
What are the benefits of the existing rule? Protecting upstream waters from pollution also protects drinking water supplies, coastal waters, and help reduce the nitrogen and phosphorus nutrient pollution which lead to dead zones.
Who opposes the existing rule? Farmers, property developers, fertilizer and pesticide producers, oil and gas producers, and golf course owners. Farmers fear that the rule would result in major costs related to environmental assessments and permits.
II. What is the goal of the proposed rule?
Rescind and re-evaluate the definition of “waters of the United States.”
The Clean Water Act of 2015 expanded the definition of “waters of the United States” to include “headwater streams, lakes, and wetlands and other waters that contribute significantly to protect the integrity of navigable waters”1 as a pollution prevention mechanism. The EPA and the Army are proposing a new rule to review and revise this definition, which is consistent with the Executive Order signed on February 28, 2017, ‘‘Restoring the Rule of Law, Federalism, and Economic Growth by Reviewing the ‘Waters of the United States’ Rule.’’
III. Leaving a Public Submission- also referred to as a “Comment”- (due September 27th, 2017) on the regulations.gov page: https://www.regulations.gov/comment?D=EPA-HQ-OW-2017-0203-0001
Do you support the proposed rule?
o Your statement should include why you support the rescinding of the definition of “waters of the United States” and reverting back to the standards that were adopted in 2008.
Do you oppose the proposed rule?
o Your statement should include why you support the current definition of “waters of the United States” and to uphold the one million comments and 1,200 peer-reviewed studies that were reviewed for the 2015 Clean Water Act.
IV. Tips on leaving a Public Submission- taken directly from the regulations.gov website[1]
· State your position (yay or nay) at the very beginning
· Avoid getting stuck in the weeds of the terminology or policy
· Base your justification on sound reasoning, scientific evidence, and/or how you will be impacted
· There is no minimum or maximum length for an effective comment
· The comment process is not a vote – one well supported comment is often more influential than a thousand form letters
V. Do agencies even read my comments?
Yes! See below from regulations.gov.
“On April 21, 2014, the agencies published a proposed rule to reduce uncertainty about the scope of “waters of the United States” covered by Clean Water Act programs, that arose from interpretation of Supreme Court decisions in 2001 and 2006, and the subsequent guidance issued by the agencies in 2008. During the public comment period, which ran until November 14, 2014, over one million comments were received. Stakeholder input received during public outreach events in combination with the written comments received during the public comment period have reshaped each of the definitions included in the final rule, ultimately with the goal of providing increased clarity for regulators, stakeholders, and the regulated public to assist them in identifying waters as “waters of the United States.” The rule reflects the judgment of the agencies when balancing the science, the statute, the Supreme Court opinions, the agencies’ expertise, and the regulatory goals of providing clarity to the public while protecting the environment and public health.”[2]
“Public participation matters. Democratic, legal, and management principles justify why public comments make a difference in regulatory policy. Public participation is an essential function of good governance. Participation enhances the quality of law and its realization through regulations (e.g. rules).”[3]
1. https://www.epa.gov/sites/production/files/2015-06/documents/508-final_clean_water_rule_economic_analysis_5-20-15.pdf
2. https://www.regulations.gov/docs/Tips_For_Submitting_Effective_Comments.pdf
[3] https://www.regulations.gov/docs/FactSheet_Public_Comments_Make_a_Difference.pdf